LUMC first in Europe to provide reprogrammed stem cells to patients receiving a kidney transplant
Illustration made by Manon Zuurmond.
&width=710&height=710)
In 2006, Japanese researcher Shinya Yamanaka revolutionized stem cell research. He discovered a method to transform ordinary body cells (such as skin and blood cells) into stem cells. These so-called induced pluripotent stem cells (iPSCs) can, like embryonic stem cells, develop into any cell type in the human body without the need for embryonic material to create them. These “magical cells” are therefore considered the holy grail of stem cell research.
Two Bags of 100 Million Cells
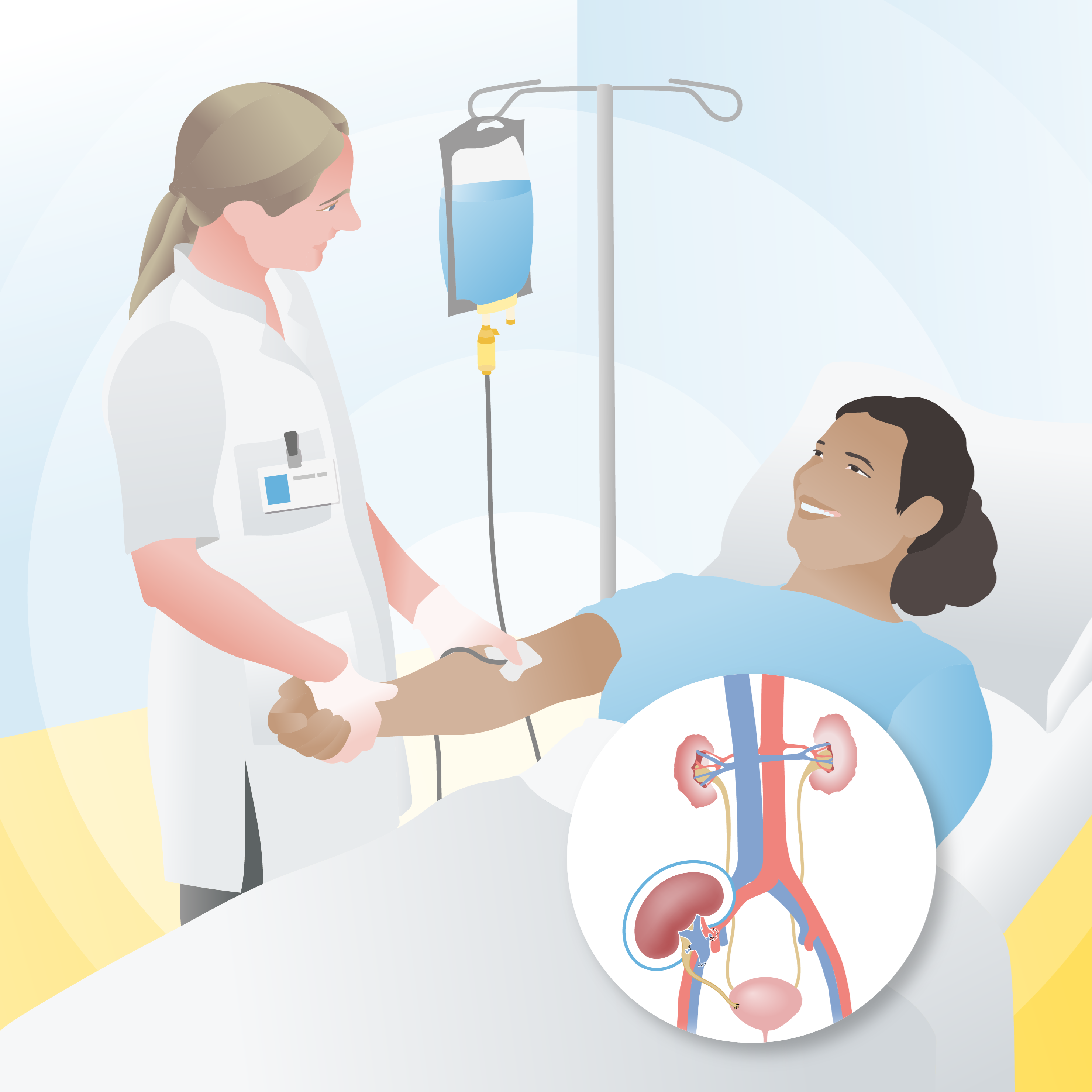
For the new cell therapy at the Leiden University Medical Center (LUMC), such iPSCs were used. Researchers have been applying stem cells in the fields of disease research and drug efficacy testing for some time, but the use of this technology in patient treatment is still rare. LUMC is a pioneer in the development and application of treatments utilizing this technology.
The iPSCs were sourced from the company Cynata Therapeutics. Through a few simple modifications, they are processed in the lab into mature cells known as mesenchymal stromal cells (MSCs). One of the properties of MSCs is their ability to instruct the immune system to slow down. “They signal the recipient’s body not to attack the new kidney so aggressively. The idea is that patients will need less anti-rejection medication to maintain the kidney,” explains lead researcher and nephrologist Siebe Spijker.
Six and seven weeks after the kidney transplant, these cells are administered to the recipient via an IV drip. Each treatment involves two bags containing about 100 million cells each.
Testing the Safety
Kidney patients will not immediately benefit from the new cell therapy. Spijker clarifies: “If we can reduce the amount of immunosuppressive medication these patients need, that would be a bonus. But that is not the aim of this study. This is a phase 1 study, primarily focused on testing the safety of this innovative therapy. The emphasis lies on the fact that this is the first time in Europe that a therapy derived from iPSCs is being used in patients with a transplanted kidney. Globally, this is only the second time kidney patients are being treated with MSCs derived from iPSCs. It’s truly unique.”
An Unlimited Supply of Stem Cells
The LUMC has the knowledge and facilities to conduct high-level stem cell research and develop innovative treatments like this one. The hospital also has a long history in kidney transplantation and has previously conducted successful studies with MSCs from bone marrow. Those cells were obtained through bone marrow puncture, a somewhat unpleasant procedure for the donor. Furthermore, due to the limited number of cells available, a donor can only help one patient per procedure.
“With iPSCs, you still need a donor initially, but afterward, you can grow an unlimited supply of stem cells in the lab and help many more patients. This offers tremendous potential,” says Spijker.
A New Kidney from Skin Cells?
Spijker considers this cell therapy a “fantastic first step” toward future stem cell treatments. “iPSCs have enormous potential. For example, we’re already using them to create mini kidneys or insulin-producing cells in the lab. In the future, we might be able to create transplantable kidneys and other organs from iPSCs. So, if you develop kidney failure, we could take one of your skin cells, turn it into a stem cell, grow it into many stem cells, and then use those to create a new kidney.”
How long it will take to generate functioning kidneys with iPSCs is hard to predict, Spijker says. “But my goal is to make it possible within my career,” says the 40-year-old nephrologist.
Difference MSCs and iPSCs
iPSCs
iPSCs are created by reprogramming normal body cells (such as skin or blood cells) into stem cells that can become any cell type: skin, bone, brain, kidneys, and so forth. These cells naturally only occur in embryos, and their properties are similar.
MSCs
iPSCs can, in turn, be programmed into MSCs. These are mature cells that naturally occur in various tissues of the body, such as bone marrow, fat tissue, and cartilage. Unlike iPSCs, MSCs can only develop into cells of a specific organ (they are multipotent). For example, after a skin wound, MSCs from the skin help generate new, healthy skin.