Parasitologie
Leiden Diagnostic Research Group
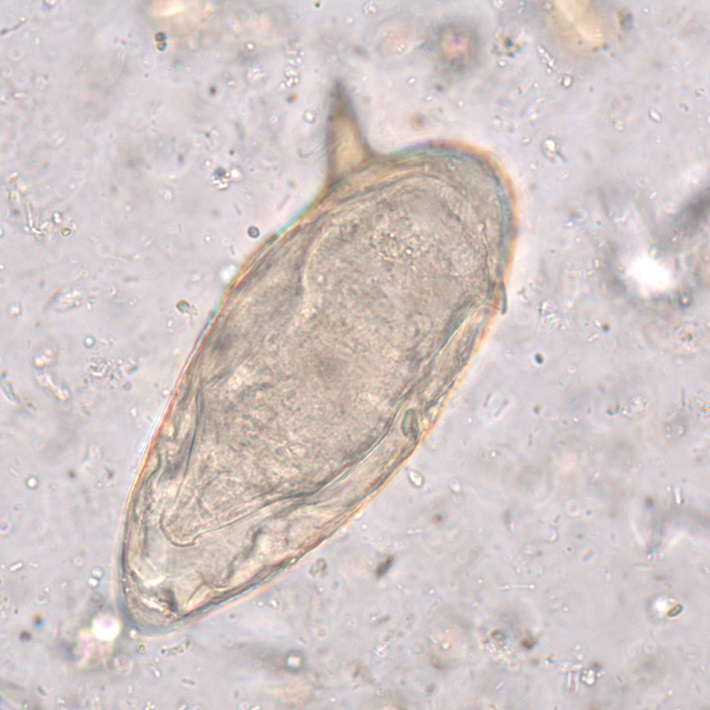
The group has a long history of diagnostic research involving the whole spectrum of human parasitic infections, but with a specific interest in schistosomiasis and intestinal helminths. These parasites are highly prevalent in low income countries where they represent a leading cause of diarrhoea, bladder and liver pathology, gynaecological disorder and anaemia. Schistosomiasis and strongyloidiasis also belong to the group of most relevant imported parasitic diseases.
Research activities of the group are related to development, implementation and evaluations of:
- immunodiagnostic assays based on the detection of Schistosoma circulating antigens, in particular CCA (Circulating Cathodic Antigen) and CAA (Circulating Anodic Antigen);
- multiplex real-time PCRs for the diagnosis of parasitic infections based on the detection of species-specific DNA in clinical samples;
- smart optical devices.
Research activities of the group are related to development, implementation and evaluations of:
- immunodiagnostic assays based on the detection of Schistosoma circulating antigens, in particular CCA (Circulating Cathodic Antigen) and CAA (Circulating Anodic Antigen);
- multiplex real-time PCRs for the diagnosis of parasitic infections based on the detection of species-specific DNA in clinical samples;
- smart optical devices.
In addition the group performs research on:
- quality assessment for the diagnosis of parasitic infections.
&width=710&height=710)
&width=710&height=710)
Projects
Team members
Departmental staff
- Dr. Lisette van Lieshout (PI)
- Eric Brienen (research technician)
Project funded staff
- Dr. Govert van Dam (senior researcher)
- Dr. Michel Bengtson (post-doc/project manager)
- Pytsje Hoekstra (PhD)