Mummery Group – Human Pluripotent Stem Cell Models of Disease
Human pluripotent stem cells (hPSCs) are derived from embryos as hESC or by reprogramming somatic tissues of the body as induced pluripotent stem cells (hiPSC, Figure 1). hiPSC capture the genome of the donor so that when derived from patients with inherited diseases, they carry genetic mutations causing the condition. We create isogenic pairs of healthy and diseased hiPSC by repairing the patient’s mutation or introducing the mutation into healthy hiPSCs or hESCs.
…Human pluripotent stem cells (hPSCs) are derived from embryos as hESC or by reprogramming somatic tissues of the body as induced pluripotent stem cells (hiPSC, Figure 1). hiPSC capture the genome of the donor so that when derived from patients with inherited diseases, they carry genetic mutations causing the condition. We create isogenic pairs of healthy and diseased hiPSC by repairing the patient’s mutation or introducing the mutation into healthy hiPSCs or hESCs.
The hPSC are differentiated to the cell types of interest and used for disease modelling, drug discovery and toxicity (Figure 2). Susceptibility to disease onset and drug sensitivity can be investigated creating new opportunities for precision (or personalized) medicine. Major advances include maturing cardiomyocytes from hiPSC by combining them with hiPSC-derived cardiac stromal cells in 3D spheroids or microtissues. This solved a major hurdle to use of hPSC-derived cardiomyocytes in academia and pharma applications and is expected to lead to widespread adoption of the technology. It is becoming increasingly evident that combining stromal cells can promote maturation of many differentiated cell types from hiPSC. We also developed open-source software called MUSCLEMOTION that allows any lab to measure contraction of cardiomyocytes in situ (as single cells, in aggregates, engineered heart tissue or even zebrafish) using movies taken simply on a smartphone (Movie 1). Most recently, hiPSC-derived cardiac and vascular cells have been combined in 3D “Organ-on-Chip” microfluidic systems to mimic vascular flow through the heart and in micro- and macro-engineered heart tissues that allow measurement of muscle contraction force. Using these technologies in combination, we have created beyond the state-of-the-art in vitro systems able to identify compounds or drugs toxic to the heart better than rodents and model diseases that are only manifest after birth or exclusive to humans. In addition, we have been able to identify specific cell types in organs like the heart that cause aspects of the disease, creating new opportunities to investigate gene therapies and how they might be implemented in Regenerative Medicine. Together, these discoveries on human PSC maturation, multicell type, human (healthy and diseased) adult-like myocardial tissues and analytical software have advanced the field such that cardiovascular disease and drug discovery is feasible in all academic and pharma laboratories.
Movie 1
Beating cardiomyocytes / heart cells derived from hPSCs.
&width=826&height=464)
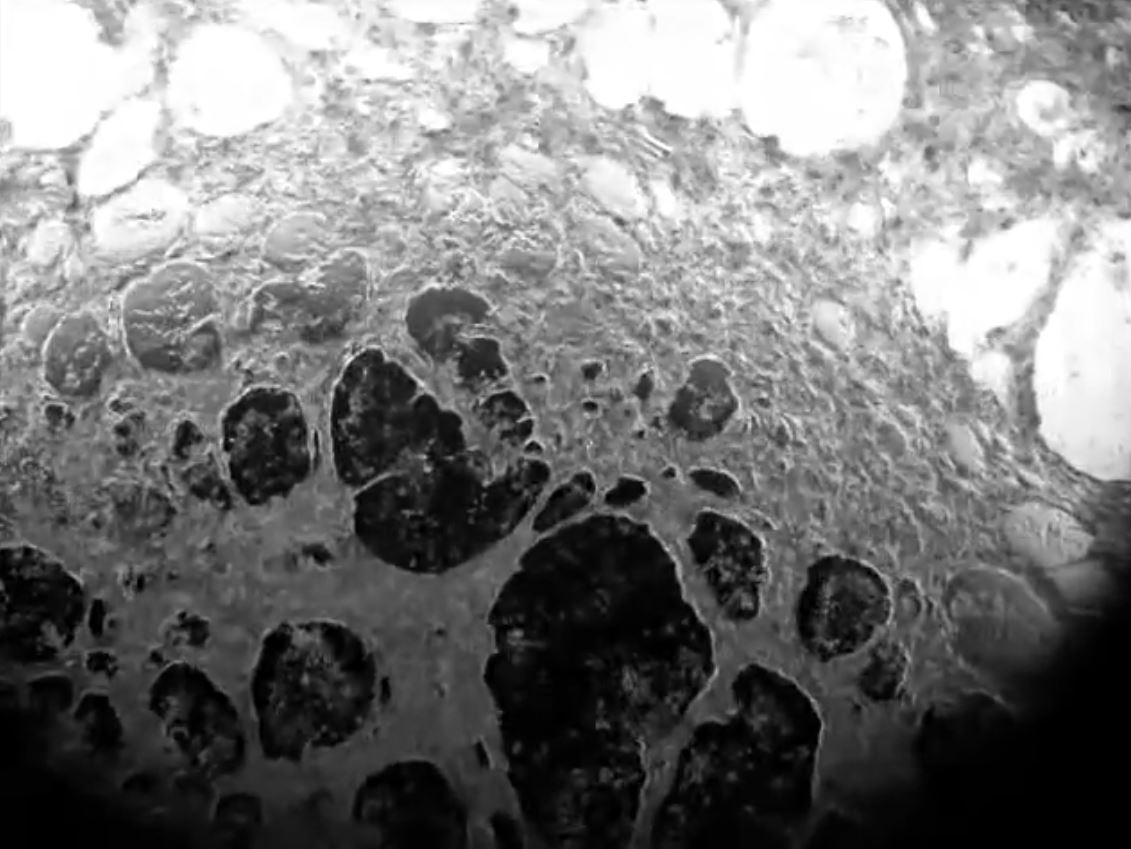
Our new method for the formation of human cardiac microtissues (as spheroids or organoids) entirely from human PSCs, is based on just 5000 cells without additional extracellular matrix components. These (inexpensive) microtissues (see project Cardiac Microtissues) contain cardiomyocytes, cardiac endothelial cells and cardiac fibroblasts derived from epicardial cells (that normally cover the surface of the heart before undergoing an endothelial to mesenchyme transition)(Figure 4, Movie 2 and Movie 3). In this model, the cardiomyocytes reach an exceptional degree of maturation, to date only seen in macroscopic engineered heart tissues (see Project Engineered Heart Tissues). Essential metabolic and paracrine signals as well as direct coupling between the cells takes place, mechanisms that cannot be mimicked by non-cardiac stromal cells. The hiPSC-derived cardiomyocytes exhibit postnatal features including T-tubules for calcium handling, highly organized sarcomeres and contraction forces, and electrophysiological properties similar to those in adult cardiomyocytes. This work is carried out in collaboration with the PI groups of Orlova, Bellin and Davis and the iPSC Hotel.
Movie 2
Milica Device
&width=826&height=464)
Movie 3
Cardiac microtissue
Microphysiological systems (MPS) include spheroids of cells (also called organoids), engineered tissues in 3D and Organs-on-Chip (usually 2-chamber devices or microchannel “chips”, that support cell growth and allow fluid flow over cell surfaces. We create tissues from stem cells in these formats because the cellular microenvironment more closely resembles that in real tissues. Engineered heart tissues (EHTs) for example are created by allowing cardiac cells to self-organize in extracellular matrix around flexible poles. As the EHTs mature, their contraction causes the poles to bend so that force can be measured. If the cells are derived from patients with (genetic forms of) heart failure, the force of contraction is lower and the poles bend less. If drugs are added that improve or interfere with contractile force, this is also measured as a change in bending of the poles. Engineered tissues can be made from any contractile muscle, such as smooth- and skeletal muscle, the cells included can be marked by reporters that allow the different cell types to be imaged. Drugs or fluids (as necessary containing inflammatory cells or cytokines) can flow through the microfluidic devices to assess responses and/or mimic infection. Some genetic diseases not only affect the heart, but also other organs like the skin , or tissues like vascular smooth muscle.
…Microphysiological systems (MPS) include spheroids of cells (also called organoids), engineered tissues in 3D and Organs-on-Chip (usually 2-chamber devices or microchannel “chips”, that support cell growth and allow fluid flow over cell surfaces. We create tissues from stem cells in these formats because the cellular microenvironment more closely resembles that in real tissues. Engineered heart tissues (EHTs) for example are created by allowing cardiac cells to self-organize in extracellular matrix around flexible poles. As the EHTs mature, their contraction causes the poles to bend so that force can be measured. If the cells are derived from patients with (genetic forms of) heart failure, the force of contraction is lower and the poles bend less. If drugs are added that improve or interfere with contractile force, this is also measured as a change in bending of the poles. Engineered tissues can be made from any contractile muscle, such as smooth- and skeletal muscle, the cells included can be marked by reporters that allow the different cell types to be imaged. Drugs or fluids (as necessary containing inflammatory cells or cytokines) can flow through the microfluidic devices to assess responses and/or mimic infection. Some genetic diseases not only affect the heart, but also other organs like the skin , or tissues like vascular smooth muscle.
This work is carried out in collaboration with the University of Twente, Hubrecht Institute, Erasmus MC, TU Delft and UMCG in the context of an NWO Gravity Grant (NOCI) and hDMT.
Movie 4
Combining just 5000 of these cells in spheroids in suspension (called microtissues or organoids) recapitulates the dialogue between blood vessels and myocardium which when disrupted can play a major role in causing heart disease.
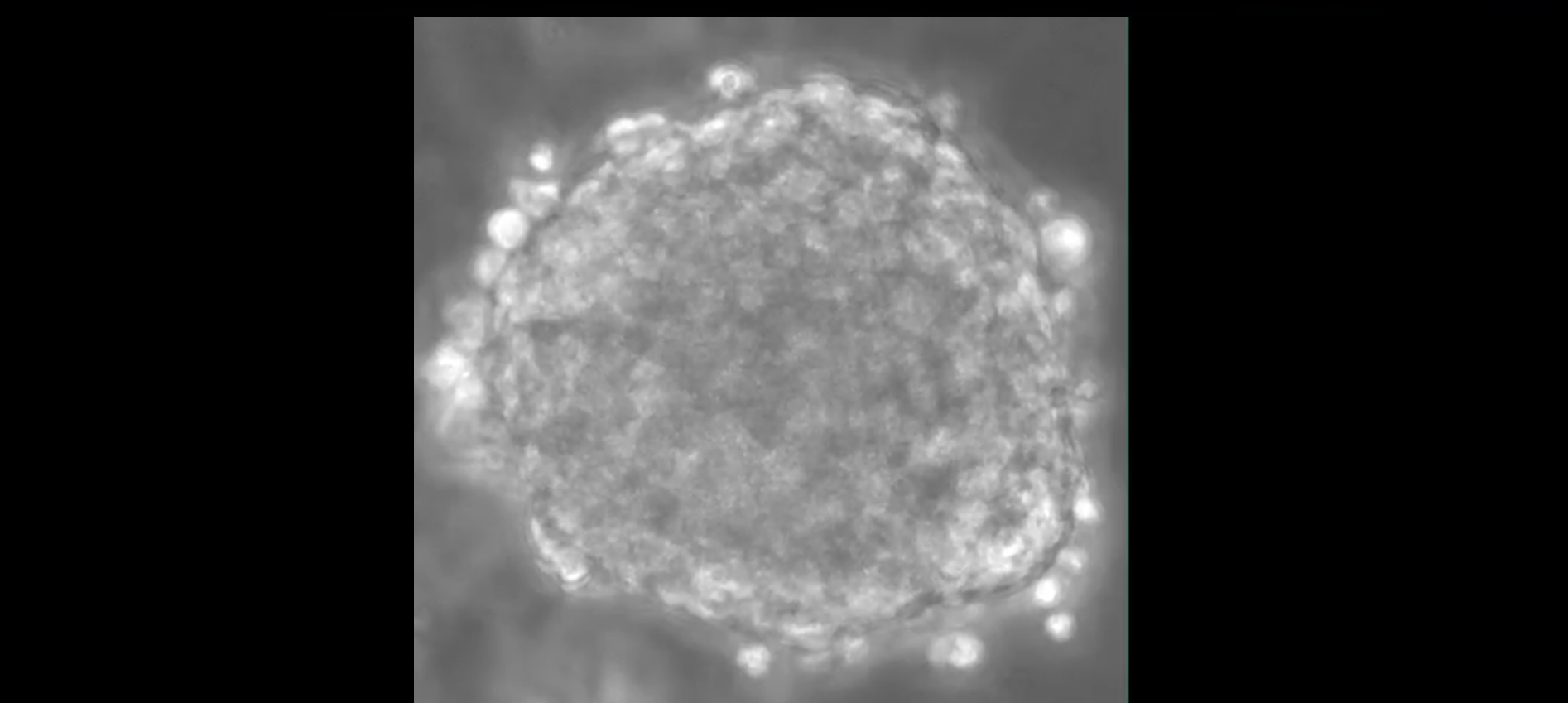&width=826&height=464)
Projects
Movie 5
Mini-organs and Organs on a Chip
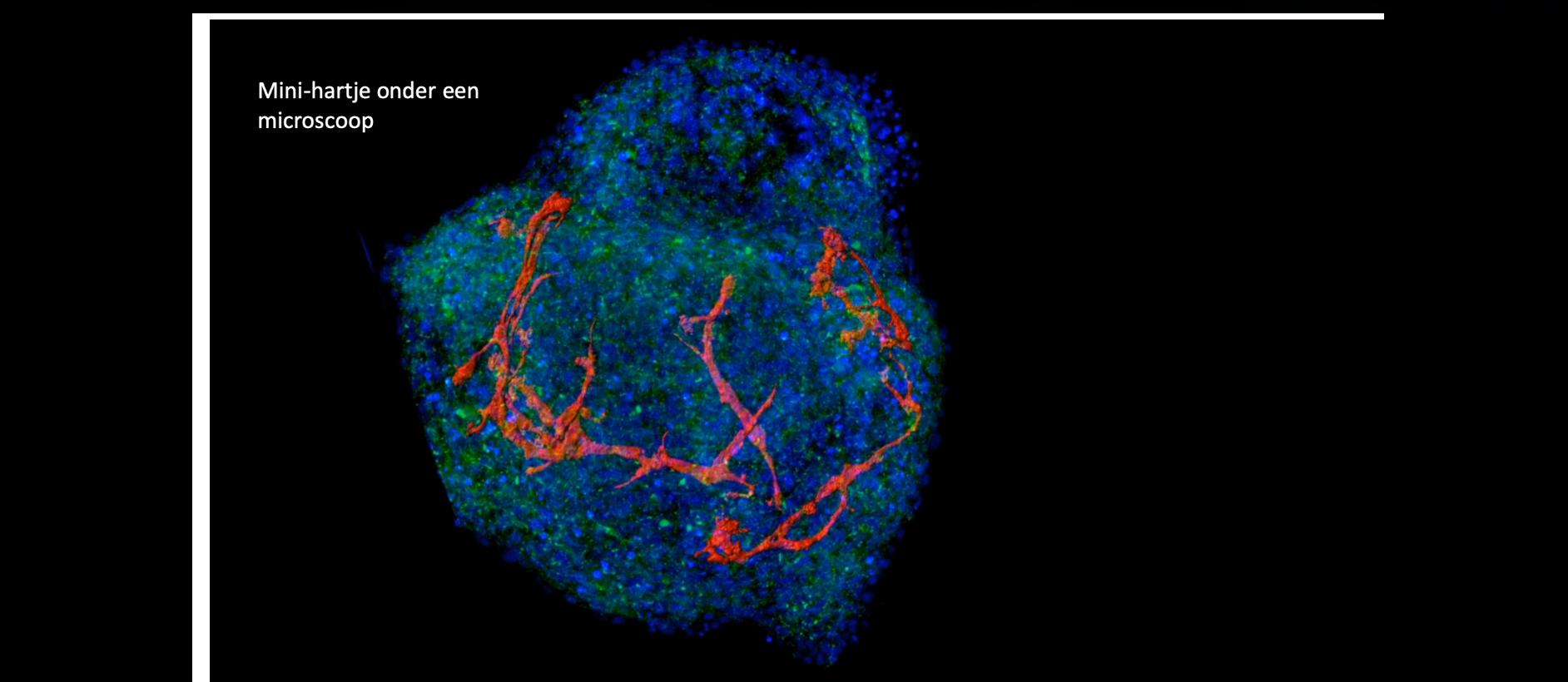&width=826&height=464)
Themes for innovation and Societal Outreach
Key publications
Our team
- Prof. Dr. Christine Mummery (Group Leader)
- Dr. Karine Raymond (Senior Researcher)
- Dr. Veronika Ramovs (Postdoc)
- Dr. Ir. Berend van Meer (Postdoc, business developer)
- Dr. Marcella Dias Brescia (Postdoc)
- Ruben van Helden (PhD student)
- Laura Windt (PhD student)
- Jeroen Stein (PhD student)
- Maury Wiendels (Research technician)

