Green Light Leiden
&width=710&height=710)
 In 2009, GLL enrolled its first patient for sentinel lymph node biopsy using Indocyanine Green (ICG), pioneering the application of fluorescence-guided techniques in clinical settings. Building on this milestone, in 2015, GLL for the first time administered a tumor-targeted imaging agent to a patient, marking another significant achievement in the clinical translation of novel imaging probes.
In 2009, GLL enrolled its first patient for sentinel lymph node biopsy using Indocyanine Green (ICG), pioneering the application of fluorescence-guided techniques in clinical settings. Building on this milestone, in 2015, GLL for the first time administered a tumor-targeted imaging agent to a patient, marking another significant achievement in the clinical translation of novel imaging probes.
Within the Academic Pharma program of the LUMC, the GLL focuses on developing and clinically translating fluorescence and other image-guided surgery applications. Our research aims to introduce clinically available imaging agents and innovate novel tumor-specific probes, ensuring safe and efficient clinical implementation.
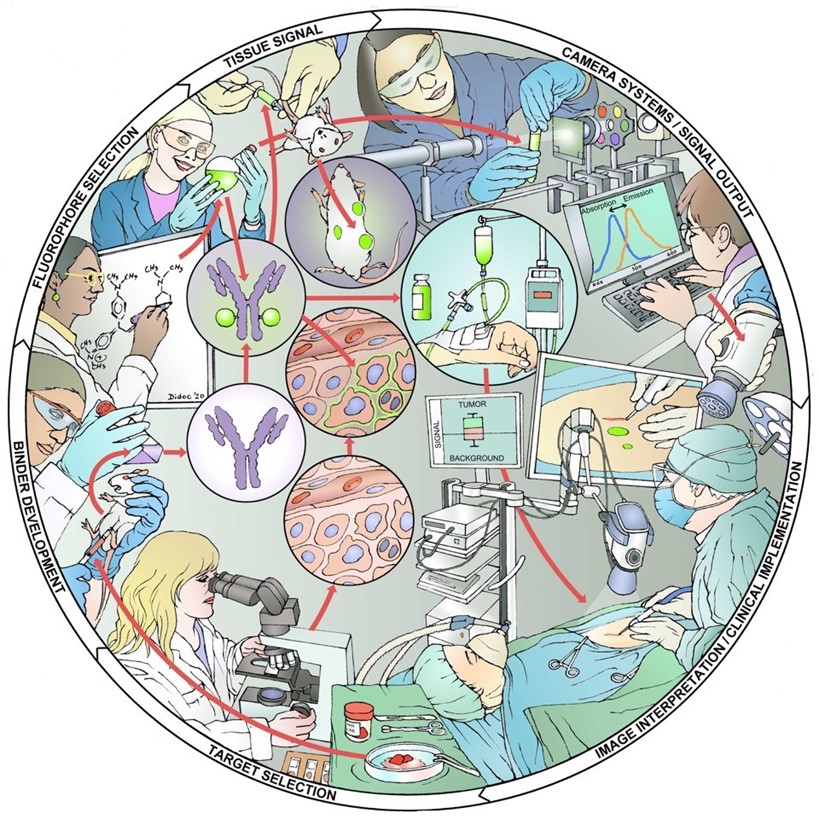
Figure 1. The Academic Pharma pipeline for image-guided surgery. Target identification, imaging agent development, preclinical validation, toxicology studies, and clinical trials.
We deliver high-quality image-guided surgery applications in partnership with leading hospitals worldwide, visualizing tissues, tumors, and critical structures with precision. Leveraging our extensive experience in (pre-)clinical development and robust clinical infrastructure, we are committed to disseminating these techniques globally.
1. Fluorescence-guided surgery
At Green Light Leiden, our mission is to revolutionize surgical precision and outcomes through the use of advanced fluorescence-guided techniques. Our research focuses on enhancing the visibility of critical anatomical structures and pathological tissues during surgery, providing surgeons with real-time, accurate information. This allows for more precise excision and spares healthy tissues. The ultimate goal is to offer patients a longer lifespan and a better quality of life. Fluorescence-guided surgery uses fluorescent imaging agents (or tracers), that can be administered locally or intravenously, and dedicated intraoperative fluorescence camera systems.
…At Green Light Leiden, our mission is to revolutionize surgical precision and outcomes through the use of advanced fluorescence-guided techniques. Our research focuses on enhancing the visibility of critical anatomical structures and pathological tissues during surgery, providing surgeons with real-time, accurate information. This allows for more precise excision and spares healthy tissues. The ultimate goal is to offer patients a longer lifespan and a better quality of life. Fluorescence-guided surgery uses fluorescent imaging agents (or tracers), that can be administered locally or intravenously, and dedicated intraoperative fluorescence camera systems.
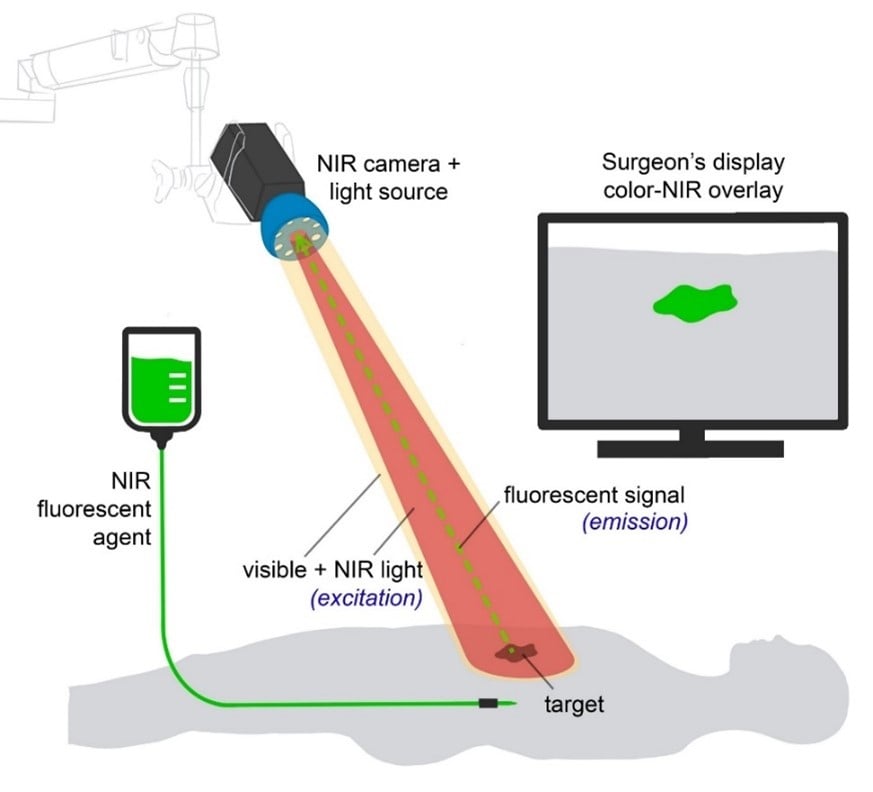
Figure 2. The basic principles of fluorescence-guided surgery. NIR fluorescent agents are administered intravenously or locally. Imaging of the agent is performed using a fluorescence imaging system. Besides a white light source and camera, this system includes a dedicated NIR excitation light, collection optics and filtration, and a camera dedicated to NIR fluorescence emission light. NIR fluorescence output is displayed on a screen in the operating theatre. A simultaneous visible light image, which can be merged with the NIR fluorescence image, is desirable. (Adapted from: Galema HA et al. Fluorescence-guided surgery in colorectal cancer; A review on clinical results and future perspectives. Eur J Surg Oncol. 2022 Apr;48(4):810-821.)
Tumor imaging
Our primary area of expertise is tumor imaging. By utilizing fluorescence-guided techniques, we can make tumor tissues light up during surgery. This allows surgeons to precisely locate and remove cancerous tissues while sparing healthy tissue, ultimately improving patient outcomes. Our techniques extend to the visualization of primary tumors, metastatic lymph nodes, and distant metastases.
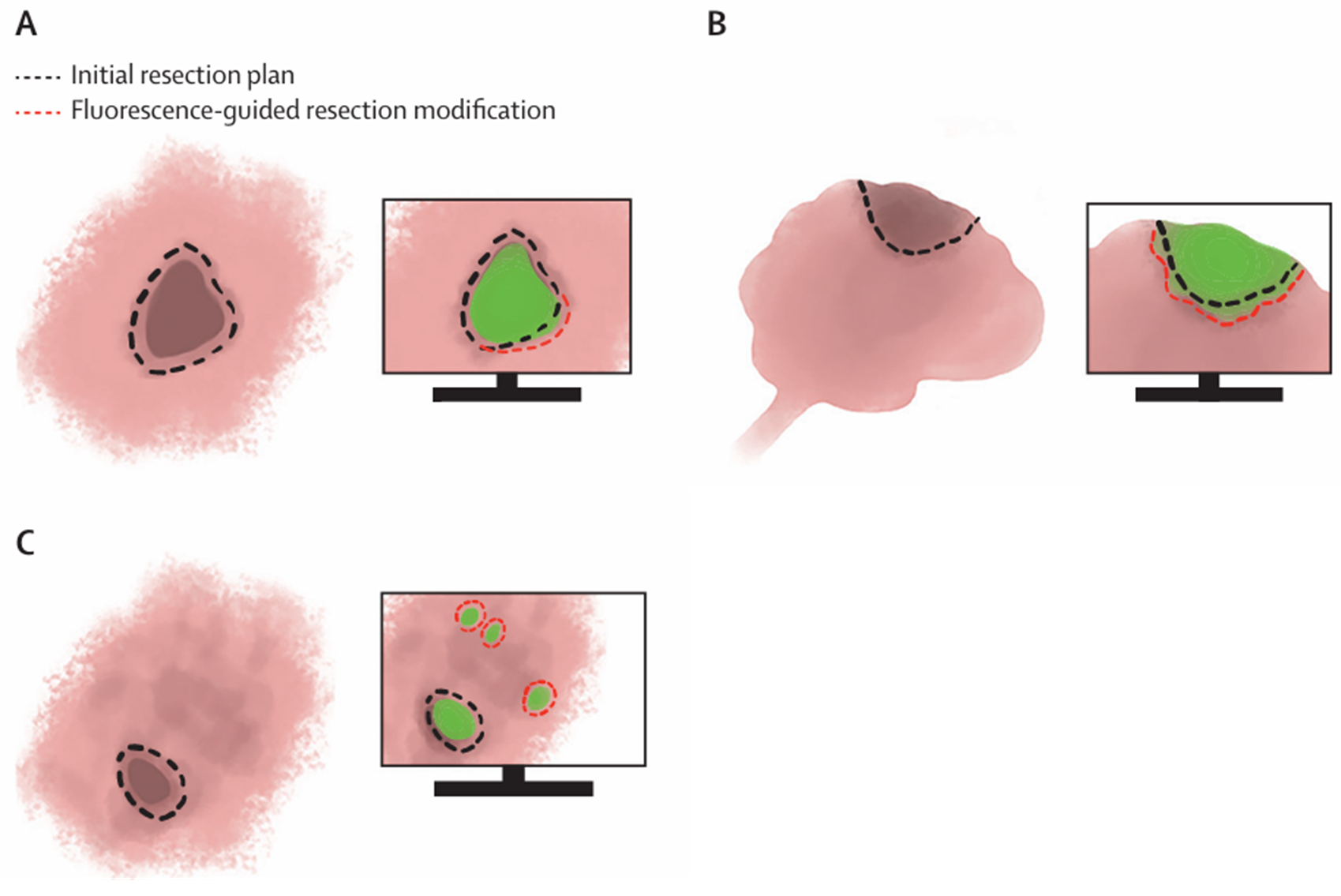
Figure 3. Schematic representations the basic applications of fluorescence-guided cancer surgery. Green shaded areas represent fluorescence emitted by tumour cells. (A) Excision with tumour-free margins. (B) Debulking procedures. (C) Identification of clinically occult lesions. (Adapted from: Lauwerends LJ et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021 May;22(5):e186-e195.)
Sentinel lymph node procedures
Another key area of expertise is sentinel lymph node procedures, which are essential for accurate cancer staging. By injecting non-specific fluorescent imaging agents such as Indocyanine Green (ICG) near the tumor site, we can effectively identify sentinel lymph nodes, the first nodes to which cancer cells are likely to spread. This technique is particularly valuable in managing breast cancer, colorectal cancer, head and neck cancer, melanoma and gynaecological tumors.
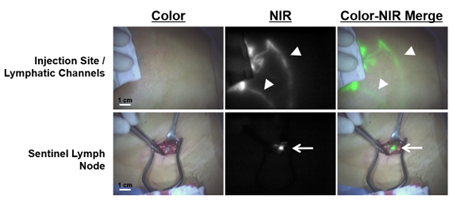
Figure 4. Fluorescence-guided sentinel lymph node biopsy in breast cancer.
Perfusion
Ensuring adequate blood flow to tissues is vital during surgery. Our fluorescence-guided techniques allow for the precise assessment of tissue perfusion in various procedures, helping to prevent complications and improve healing. Applications include: gastro-intestinal anastomoses, vascular surgery on extremities, plastic surgery reconstructions, trauma surgery for fractures, liver surgery and lung surgery.
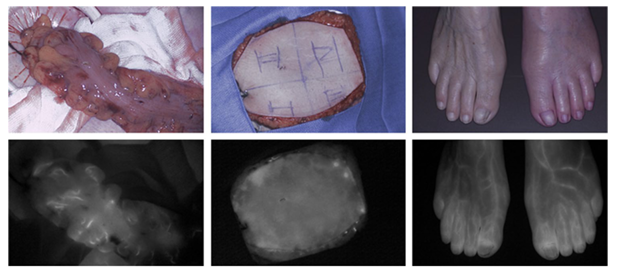
Figure 5. Perfusion assessment of the colon (left), a free-flap for reconstructive breast surgery (middle), and the lower extremities (right) using ICG.
Visualizing vital structures
Avoiding damage to vital structures during surgery is critical for patient safety and recovery. Fluorescence-guided imaging helps surgeons clearly see important structures such as the ureters, the biliary tract and nerves.
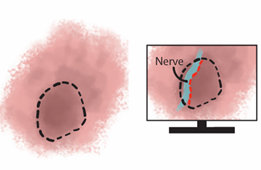
Figure 6. Schematic representation of the fluorescence-guided identification of vital structures (blue shaded area represents fluorescence emitted by nerve). (Adapted from: Lauwerends LJ et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021 May;22(5):e186-e195.)
2. Targeting cancer – Academic Pharma
At Green Light Leiden, we are also dedicated to advancing the field of image-guided surgery and cancer treatment through our collaboration within the Academic Pharma program of the LUMC (Academic Pharma | LUMC). This program represents a synergy between cutting-edge research and clinical application, aiming to bring innovative cancer treatments from the lab to the patient. Our Academic Pharma program encompasses a comprehensive pipeline that ensures each step, from discovery to clinical application, is meticulously managed:
…At Green Light Leiden, we are also dedicated to advancing the field of image-guided surgery and cancer treatment through our collaboration within the Academic Pharma program of the LUMC (Academic Pharma | LUMC). This program represents a synergy between cutting-edge research and clinical application, aiming to bring innovative cancer treatments from the lab to the patient. Our Academic Pharma program encompasses a comprehensive pipeline that ensures each step, from discovery to clinical application, is meticulously managed:
- Target Identification: Our research begins with identifying new molecular targets for imaging and cancer treatment. This involves understanding the specific markers and pathways associated with different cancer types.
- Imaging Agent Development: Once targets are identified, we develop novel imaging agents that can specifically bind to these targets. These agents are designed to improve the visualization of cancer cells during surgery, enhancing the accuracy of tumor excision.
- Preclinical Validation: Before our imaging agents can be tested in humans, we conduct extensive preclinical validation. This includes testing in cell cultures and animal models to ensure efficacy and safety.
- Toxicology Studies: Safety is paramount. We perform rigorous toxicology studies to determine the potential side effects and safety profile of our imaging agents, ensuring they are safe for human use.
- Clinical Trials (Phase I-III): Our agents undergo a series of clinical trials to test their safety, efficacy, and optimal usage in humans. These trials are conducted in phases, starting with small groups of patients and expanding to larger populations.
By combining cutting-edge research with clinical application, Green Light Leiden aims to lead the way in improving surgical outcomes and advancing cancer treatment. Our commitment to innovation and excellence ensures that patients receive the most effective and safe treatments possible.
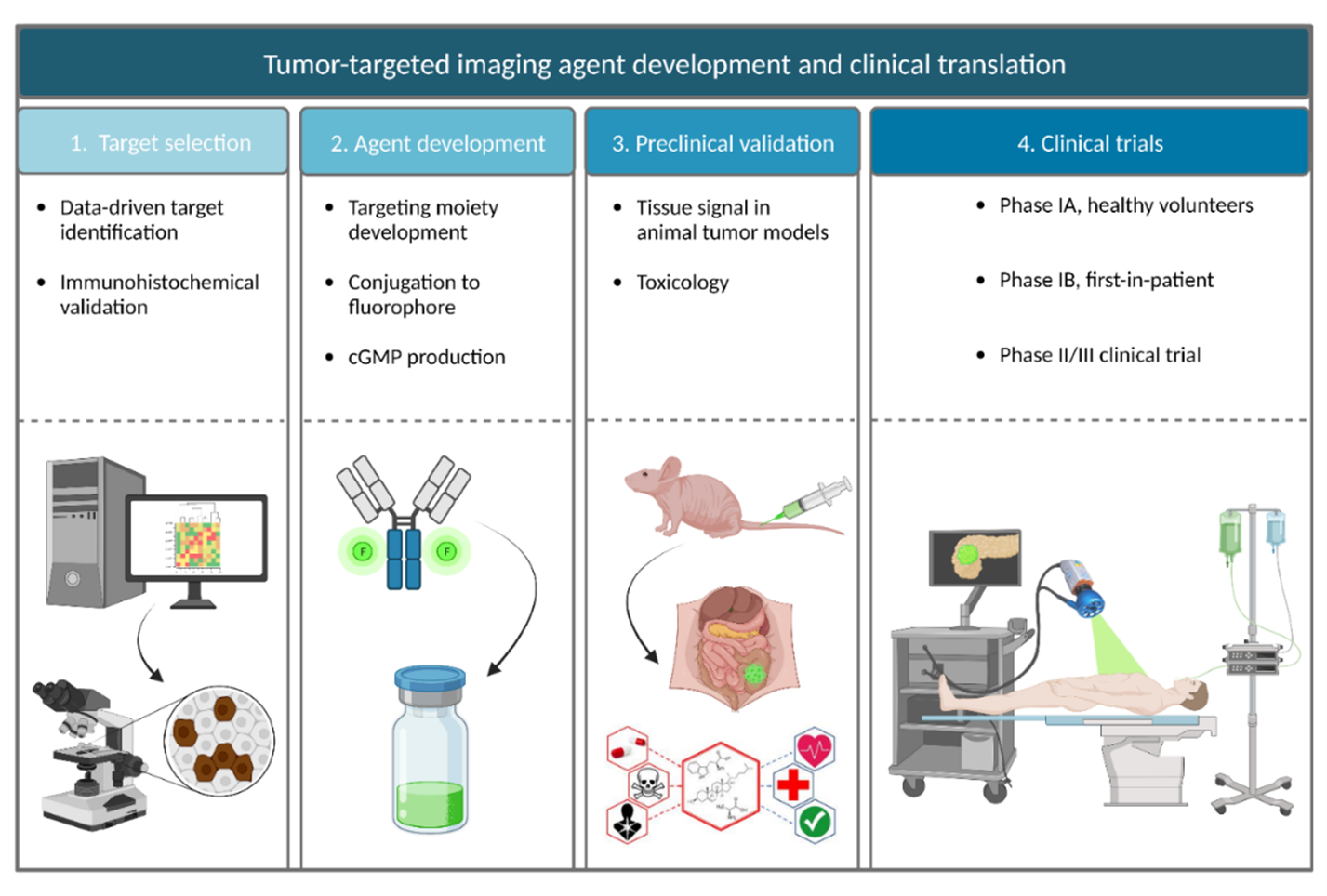
Figure 7. Schematic overview of the different phases in the development and clinical translation of tumor-targeted imaging agents. (Adapted from: Linders DGJ et al. Tumor-targeted precision surgery; SPIE: 2023; Vol. 12361.).
3. Combining Image-Guided Surgery applications
At Green Light Leiden, we are at the forefront of integrating various advanced technologies to further enhance the precision and effectiveness of image-guided surgery.
3D Modelling and the Surgical Cockpit
We use 3D modelling to create detailed anatomical maps based on preoperative imaging. These models help surgeons plan complex procedures and give intraoperative guidance by providing a comprehensive view of the surgical field, which improves accuracy and outcomes. The Surgical Cockpit is an in-house developed multimodality imaging platform integrating intraoperative 3D modelling, ultrasound, and fluorescence imaging into the robotic surgical console. It offers surgeons a centralized, real-time assessment of the surgical field by integration of multiple imaging modalities into one single view.
…At Green Light Leiden, we are at the forefront of integrating various advanced technologies to further enhance the precision and effectiveness of image-guided surgery.
3D Modelling and the Surgical Cockpit
We use 3D modelling to create detailed anatomical maps based on preoperative imaging. These models help surgeons plan complex procedures and give intraoperative guidance by providing a comprehensive view of the surgical field, which improves accuracy and outcomes. The Surgical Cockpit is an in-house developed multimodality imaging platform integrating intraoperative 3D modelling, ultrasound, and fluorescence imaging into the robotic surgical console. It offers surgeons a centralized, real-time assessment of the surgical field by integration of multiple imaging modalities into one single view.
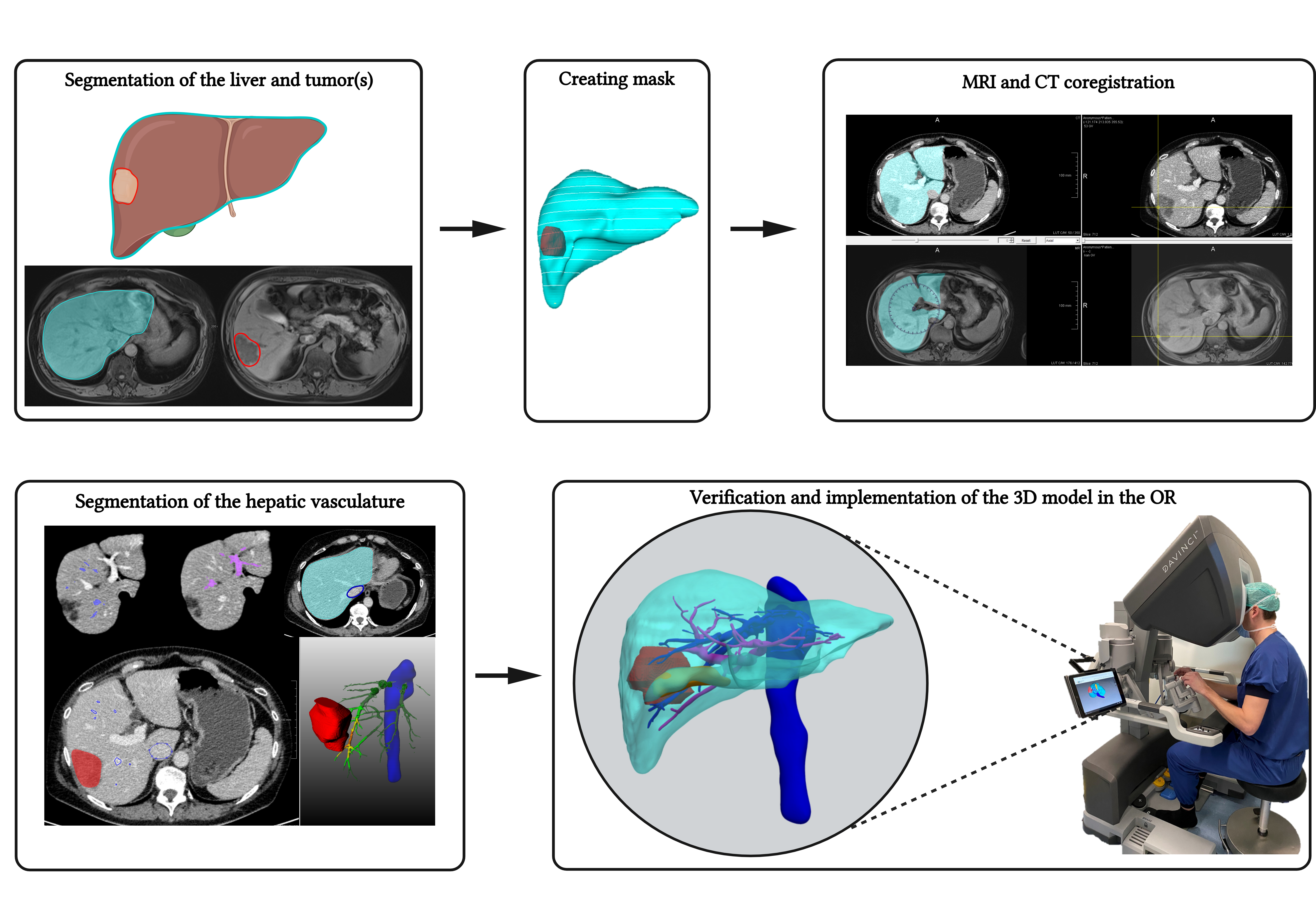 Figure 8. Schematic overview of the segmentation process. (Adapted from: Bijlstra OD et al. Integration of Three-Dimensional Liver Models in a Multimodal Image-Guided Robotic Liver Surgery Cockpit. Life 2022, 12, 667.).
Figure 8. Schematic overview of the segmentation process. (Adapted from: Bijlstra OD et al. Integration of Three-Dimensional Liver Models in a Multimodal Image-Guided Robotic Liver Surgery Cockpit. Life 2022, 12, 667.).
Intraoperative Ultrasound
Intraoperative ultrasound provides real-time imaging during surgery, allowing for immediate adjustments and verification of tissue conditions. This tool is essential for identifying and avoiding critical structures, as well as for assessing tumor margins.
Tumor-targeted PET Imaging
Positron Emission Tomography (PET) imaging, when combined with tumor-targeted agents, allows for the precise localization of cancerous tissues and improved neoadjuvant treatment response evaluation compared with conventional anatomical imaging modalities.
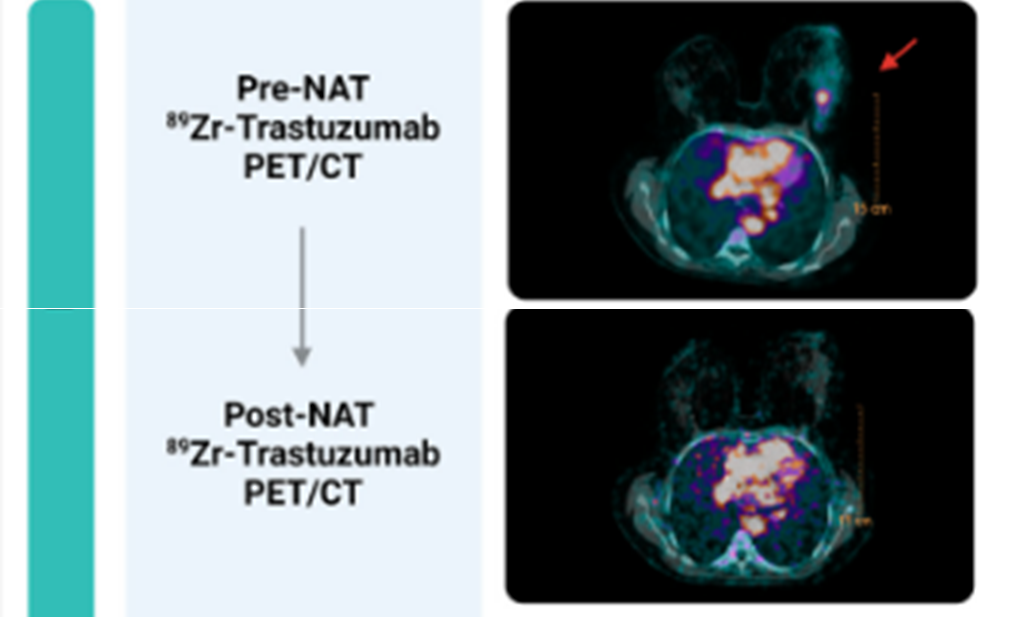
Figure 9. 89Zr-trastuzumab-PET/CT for neoadjuvant treatment response evaluation in HER2-positive breast cancer. (Adapted from: Linders DGJ et al. 89Zr-Trastuzumab PET/CT Imaging of HER2-Positive Breast Cancer for Predicting Pathological Complete Response after Neoadjuvant Systemic Therapy: A Feasibility Study. Cancers. 2023, 15, 4980.)
Hybrid Imaging and SPECT
We use Single Photon Emission Computed Tomography (SPECT) with dual-(radio and fluorescently) labeled tracers. Such tracers enable both SPECT/CT imaging to assess tumor and lymph node status preoperatively and intraoperative fluorescence imaging and radiodetection. After administration, SPECT/CT images map the primary tumor and lymph nodes. During surgery, a gamma probe helps localize deep-seated tumors and lymph nodes, while near-infrared fluorescence imaging aids in visualizing tumor margins and identifying residual disease. This dual approach enhances surgical precision, aiding in the accurate removal of cancerous tissues while preserving healthy structures.
Laser Speckle
Laser speckle imaging is a non-invasive technique that provides real-time visualization of blood flow and tissue perfusion during surgery. By analyzing the speckle pattern produced by laser light scattering off moving red blood cells, surgeons can assess the viability of tissues and ensure adequate perfusion. This method is particularly valuable in procedures where maintaining proper blood flow is critical, such as in reconstructive surgeries and flap viability assessments. Laser speckle imaging helps in making immediate decisions about tissue health, thereby improving surgical outcomes and patient recovery.
Hyperspectral Imaging
Hyperspectral imaging captures a wide spectrum of light to provide detailed information about tissue composition. This technology helps differentiate between healthy and diseased tissues more accurately.
Remote Monitoring
Remote monitoring systems enable real-time patient oversight by experts who may not be physically present. It can be used for the early detection of complications after surgery and to closely monitor the patient’s recovery, for example on the hospital ward or at home.
Artificial Intelligence Applications in Surgery
Artificial Intelligence (AI) is revolutionizing surgery by providing advanced analytical tools that can predict outcomes, suggest surgical strategies, and assist in real-time decision-making. AI algorithms analyze vast amounts of data to help surgeons achieve better precision and outcomes.
4. Clinical Trials
Ongoing
-
- FLUOPANC-II: A performance study of SGM-101, a fluorochrome-labeled anti-CEA monoclonal antibody for fluorescence-guided imaging to determine local extent and resectability during surgical resection of pancreatic ductal adenocarcinoma after neoadjuvant treatment.
- TRIPHASE-Trial: A Phase 2/3 randomized controlled semi-blinded trial to investigate safety and effectiveness in visualization of the ureters with nizaracianine triflutate, administered intravenously in up to three divided doses, in patients undergoing abdominopelvic surgical procedures
- FOCUS-GREEN-Trial: Dual dye fluorescence-guided resection of colorectal liver metastases using indocyanine green (ICG) and the CEA-targeted fluorescent agent SGM-101
- SGM-LARRC-Trial: Multicenter, open-label, controlled, parallel arms clinical study on the performance of SGM-101, a fluorochrome-labeled anti-carcino-embryonic antigen (CEA) monoclonal antibody, for locally advanced or recurrent rectal cancer patients undergoing curative surgery.
- MIMIC-II-Trial: This study investigates the optimal dose and timing of ICG for use in surgery of colorectal liver metastases. The study specifically focuses on patients who have been pretreated with chemotherapy, as previous data has shown that in these patients, ICG accumulates less effectively and therefore requires further optimization.
- SELECT-Trial: Selective ICG injection of a segmental hepatic artery followed by NIR fluorescence-guided anatomical liver resection: a feasibility study
- SGM-Phase III-Trial: Multicenter, semi-blinded, randomized, controlled, parallel arms clinical study on the performance of SGM-101, a fluorochrome-labeled anti-carcino-embryonic antigen (CEA) monoclonal antibody, for the delineation of primary and recurrent tumor and metastases in patients undergoing curative surgery for colorectal cancer.
- SGM-T1-Trial: CEA-targeted fluorescence endoscopy to enable discrimination of malignant from benign tissue in rectal polyps with suspected T1 adenocarcinoma or high grade dysplasia: a feasibility study
- SGM-CBM Trial: A feasibility study of SGM-101, a fluorochrome-labeled anti-CEA targeted monoclonal antibody for the intra-operative detection of colorectal brain metastases.
- TESTPANC-Trial: Evaluate the feasibility of a 3D culture platform for testing therapeutic response in pancreatic cancer on patient derived tissues.
- INFLOW-study: A study to improve the effectiveness of percutaneous transluminal angioplasty success in patients with peripheral artery disease
- IMPACT-study: A feasibility study to assess the potential of ICG in quantifying perfusion in patients with traumatic bone or soft tissue injuries
- FAFI-study: A multicentre randomized controlled trial to assess whether intraoperative ICG reduces the fat necrosis incidence in reconstructive DIEP-flap breast surgery.
- PHOOTONICS-study: The use of multispectral imaging as a new technique to help monitor and prevent foot ulcers in diabetic patients.
- Revascularisation cohort: A prediction of the effectiveness of a revascularization procedure in patients with peripheral arterial disease through intravenous administration of ICG before and after the procedure.
- Diabetes study: A study to assess the influence of Diabetes Mellitus on the perfusion of the lower extremities in patients suffering from peripheral arterial disease.
- Multicentre amputation study: Prediction of wound healing potential after a lower extremity amputation in patients with a high risk of wound healing disorders.
- Sigmoid perfusion in open abdominal aortic aneurysm repair: A study into perfusion assessment of the colon to prevent and help monitor ischemia in open abdominal aortic aneurysm repair.
- Colon cohort: A study to predict and reduce anastomotic leakage after colon resection surgery.
- Gastric conduit cohort: A study to predict and reduce anastomotic leakage after gastric conduit surgery in oesophageal cancer patients.
- Myocardial perfusion study: This study will focus on the assessment of myocardial perfusion changes after placement of the coronary artery bypass graft
Ongoing
-
- FLUOPANC-II: A performance study of SGM-101, a fluorochrome-labeled anti-CEA monoclonal antibody for fluorescence-guided imaging to determine local extent and resectability during surgical resection of pancreatic ductal adenocarcinoma after neoadjuvant treatment.
- TRIPHASE-Trial: A Phase 2/3 randomized controlled semi-blinded trial to investigate safety and effectiveness in visualization of the ureters with nizaracianine triflutate, administered intravenously in up to three divided doses, in patients undergoing abdominopelvic surgical procedures
- FOCUS-GREEN-Trial: Dual dye fluorescence-guided resection of colorectal liver metastases using indocyanine green (ICG) and the CEA-targeted fluorescent agent SGM-101
- SGM-LARRC-Trial: Multicenter, open-label, controlled, parallel arms clinical study on the performance of SGM-101, a fluorochrome-labeled anti-carcino-embryonic antigen (CEA) monoclonal antibody, for locally advanced or recurrent rectal cancer patients undergoing curative surgery.
- MIMIC-II-Trial: This study investigates the optimal dose and timing of ICG for use in surgery of colorectal liver metastases. The study specifically focuses on patients who have been pretreated with chemotherapy, as previous data has shown that in these patients, ICG accumulates less effectively and therefore requires further optimization.
- SELECT-Trial: Selective ICG injection of a segmental hepatic artery followed by NIR fluorescence-guided anatomical liver resection: a feasibility study
- SGM-Phase III-Trial: Multicenter, semi-blinded, randomized, controlled, parallel arms clinical study on the performance of SGM-101, a fluorochrome-labeled anti-carcino-embryonic antigen (CEA) monoclonal antibody, for the delineation of primary and recurrent tumor and metastases in patients undergoing curative surgery for colorectal cancer.
- SGM-T1-Trial: CEA-targeted fluorescence endoscopy to enable discrimination of malignant from benign tissue in rectal polyps with suspected T1 adenocarcinoma or high grade dysplasia: a feasibility study
- SGM-CBM Trial: A feasibility study of SGM-101, a fluorochrome-labeled anti-CEA targeted monoclonal antibody for the intra-operative detection of colorectal brain metastases.
- TESTPANC-Trial: Evaluate the feasibility of a 3D culture platform for testing therapeutic response in pancreatic cancer on patient derived tissues.
- INFLOW-study: A study to improve the effectiveness of percutaneous transluminal angioplasty success in patients with peripheral artery disease
- IMPACT-study: A feasibility study to assess the potential of ICG in quantifying perfusion in patients with traumatic bone or soft tissue injuries
- FAFI-study: A multicentre randomized controlled trial to assess whether intraoperative ICG reduces the fat necrosis incidence in reconstructive DIEP-flap breast surgery.
- PHOOTONICS-study: The use of multispectral imaging as a new technique to help monitor and prevent foot ulcers in diabetic patients.
- Revascularisation cohort: A prediction of the effectiveness of a revascularization procedure in patients with peripheral arterial disease through intravenous administration of ICG before and after the procedure.
- Diabetes study: A study to assess the influence of Diabetes Mellitus on the perfusion of the lower extremities in patients suffering from peripheral arterial disease.
- Multicentre amputation study: Prediction of wound healing potential after a lower extremity amputation in patients with a high risk of wound healing disorders.
- Sigmoid perfusion in open abdominal aortic aneurysm repair: A study into perfusion assessment of the colon to prevent and help monitor ischemia in open abdominal aortic aneurysm repair.
- Colon cohort: A study to predict and reduce anastomotic leakage after colon resection surgery.
- Gastric conduit cohort: A study to predict and reduce anastomotic leakage after gastric conduit surgery in oesophageal cancer patients.
- Myocardial perfusion study: This study will focus on the assessment of myocardial perfusion changes after placement of the coronary artery bypass graft
Recently Completed
-
- FLUOPANC-I: A Phase II feasibility trial for intraoperative near-infrared fluorescence imaging in pancreatic- and extrahepatic bile duct tumors using cRGD-ZW800-1
- FLURETER-Study: A phase 1 trial focusing on the safety, efficacy, and pharmacokinetics of Nizaracianine, a fluorescent probe for ureter imaging
- HER2-PET Study: treatment response evaluation after neoadjuvant systemic therapy in patients with HER2-positive breast cancer using tumor-targeted PET/CT.
Future
-
- CRITICAL-Trial: An open-label, exploratory phase II study to assess the efficacy of cRGD-ZW800-1 in intraoperative tumor-specific near-infrared fluorescence imaging of non-small cell lung cancer and lung metastases of various origins
- Neo-RGD-Trial: an open-label, exploratory phase II study to assess the efficacy of cRGD-ZW800-1 after neo-adjuvant therapy in tumor-specific near-infrared fluorescence imaging of rectal and sigmoid carcinoma
- SPRAYDYE-Trial: A phase I/II study to assess the efficacy, safety and tolerability of a topically applied, cathepsin-activatable fluorescent imaging agent, for real-time intraoperative resection margin assessment in breast conserving surgery
5. The Green Light Leiden Team
Principal Investigators
- Alexander Vahrmeijer – Founder Green Light Leiden
- Sven Mieog – Green Light HPB and Surgical Oncology
- Joost van der Voorst – Green Light Vascular
- Denise Hilling – Green Light Artificial Intelligence
- Peter Kuppen – Green Light Preclinical
- Koos Burggraaf – Center for Human Drug Research
- Stefan Harmsen – Probe Development
Principal Investigators
- Alexander Vahrmeijer – Founder Green Light Leiden
- Sven Mieog – Green Light HPB and Surgical Oncology
- Joost van der Voorst – Green Light Vascular
- Denise Hilling – Green Light Artificial Intelligence
- Peter Kuppen – Green Light Preclinical
- Koos Burggraaf – Center for Human Drug Research
- Stefan Harmsen – Probe Development
Full-time PhD-candidates
- Cedric Pesch, MD
- Mats Warmerdam, MD
- Mo Kruiswijk, BSc
- Roderick Peul, BSc
- Rutger Henrar, MD
- Stefan Koning, MD
- Tom Dijkhuis, MSc
- Tom van Ravens, MD
- Ana Matoshi, MSc
- Elham Zonoobi, MSc
- Lizzie de Muynck, MSc
- Nada Badr, MSc
Clinicians finalizing PhD-thesis
- Daan Linders, MD
- Lisanne Neijenhuis, MD
- Martijn van Dam, MD
- Floris Tange, BSc
- Fokkedien Tummers, MD
- Friso Achterberg, MD
- Judith Stibbe, MD
- Marion Deken, MD
- Okker Bijlstra, MD
- Robin Faber, MD
- Ruben Houvast, MSc
- Ruben Meijer, MD
Preclinical researchers
- Geeske Dekker – Ensink
- Ronald van Vlierberghe
- Shadhvi Bhairosingh
Green Light Leiden Alumni
- Merlijn Hutteman
- Sven Mieog
- Joost van der Vorst
- Floris Verbeek
- Martin Boonstra
- Bob Schaafsma
- Quirijn Tummers
- Hein Handgraaf
- Marieke Stammes
- Charlotte Hoogstins
- Pieter van Driel
- Willemieke Tummers
- Noor Boogerd
- Babs Sibinga Mulder
- Paulien Stegehuis
- Pim van den Hoven
- Kim de Valk
- Lianne Wellens
- Thomas Brouwer
- Victor Baart
- Labrinus van Manen
- Floris Vuijk
6. Collaborators
Our success at Green Light Leiden is built on strong collaborations with renowned institutions and leading academic experts worldwide. These partnerships enhance our research capabilities and expand the impact of our innovative techniques. We believe hat through a combined effort we can accomplish more.
Internal collaborations within the LUMC
Within the LUMC, we collaborate with multiple departments, including the Departments of Oncologic and Vascular Surgery, Thoracic Surgery, Neurosurgery, Head and Neck Surgery, Gynaecology, Urology, Pathology, Radiology, Nephrology, Clinical Pharmacology, Chemical Immunology and the Chemical Signaling Lab. We also work closely with the Academic Pharma and GMP Facility.
…Our success at Green Light Leiden is built on strong collaborations with renowned institutions and leading academic experts worldwide. These partnerships enhance our research capabilities and expand the impact of our innovative techniques. We believe hat through a combined effort we can accomplish more.
Internal collaborations within the LUMC
Within the LUMC, we collaborate with multiple departments, including the Departments of Oncologic and Vascular Surgery, Thoracic Surgery, Neurosurgery, Head and Neck Surgery, Gynaecology, Urology, Pathology, Radiology, Nephrology, Clinical Pharmacology, Chemical Immunology and the Chemical Signaling Lab. We also work closely with the Academic Pharma and GMP Facility.
Local Collaborations in Leiden
Our local partnerships include the Centre for Human Drug Research (CHDR), with whom we collaborate closely for all first-in human phase I studies. Additionally we collaborate with the Leiden Academic Centre for Drug Research (LACDR), specializing in computational modeling.
National
Our national collaborators help us extend our research and clinical applications across the Netherlands. We work together with clinicians and researcher of (among others) Amsterdam University Medical Center (AUMC), Netherlands Cancer Institute (NKI), Princess Maxima Center for Pediatric Oncology, Catharina Hospital Eindhoven, Erasmus University Medical Center Rotterdam, University Medical Center Groningen (UMCG), Radboud University Medical Center, Haaglanden Medical Center (HMC), and Alrijne Hospital.
International
Our research also benefits from international collaborations with prestigious institutions, including Stanford University, Harvard University, Case Western Reserve University, University of Pennsylvania, University College Dublin, Max Planck Institute for Experimental Medicine Göttingen, Purdue University, Free University Brussels, and University of Strasbourg.
Companies
We work with leading companies to integrate the latest technological advancements into our research and clinical practices: Diagnostic Green, Philips Healthcare, Quest/Olympus, Karl-Storz, SurgiMab, and Curadel. Our collaborative approach ensures that we stay at the forefront of medical innovation, combining expertise from academia, healthcare, and industry to improve patient outcomes.
Join Us
At Green Light Leiden, we believe in the power of collaboration. We are always looking to expand our network of partners to further advance our mission. Become our next collaboration partner and join us in our journey towards brighter, healthier futures for patients worldwide.
7. Key Publications
- Robin A Faber, Ruben P J Meijer, Daphne H M Droogh, Jasmijn J Jongbloed, Okker D Bijlstra, Fran Boersma, et al. Indocyanine green near-infrared fluorescence bowel perfusion assessment to prevent anastomotic leakage in minimally invasive colorectal surgery (AVOID): a multicentre, randomised, controlled, phase 3 trial, The Lancet Gastroenterology & Hepatology, 2024.
- Achterberg FB, Bijlstra OD, Slooter MD, Sibinga Mulder BG, Boonstra MC, Bouwense SA, Bosscha K, Coolsen MME, Derksen WJM, Gerhards MF, Gobardhan PD, Hagendoorn J, Lips D, Marsman HA, Zonderhuis BM, Wullaert L, Putter H, Burggraaf J, Mieog JSD, Vahrmeijer AL, Swijnenburg RJ; Dutch Liver Surgery Group. ICG-Fluorescence Imaging for Margin Assessment During Minimally Invasive Colorectal Liver Metastasis Resection. JAMA Netw Open. 2024 Apr 1;7(4):e246548.
…- Robin A Faber, Ruben P J Meijer, Daphne H M Droogh, Jasmijn J Jongbloed, Okker D Bijlstra, Fran Boersma, et al. Indocyanine green near-infrared fluorescence bowel perfusion assessment to prevent anastomotic leakage in minimally invasive colorectal surgery (AVOID): a multicentre, randomised, controlled, phase 3 trial, The Lancet Gastroenterology & Hepatology, 2024.
- Achterberg FB, Bijlstra OD, Slooter MD, Sibinga Mulder BG, Boonstra MC, Bouwense SA, Bosscha K, Coolsen MME, Derksen WJM, Gerhards MF, Gobardhan PD, Hagendoorn J, Lips D, Marsman HA, Zonderhuis BM, Wullaert L, Putter H, Burggraaf J, Mieog JSD, Vahrmeijer AL, Swijnenburg RJ; Dutch Liver Surgery Group. ICG-Fluorescence Imaging for Margin Assessment During Minimally Invasive Colorectal Liver Metastasis Resection. JAMA Netw Open. 2024 Apr 1;7(4):e246548.
- Michiels N, Doppenberg D, Groen JV, van Veldhuisen E, Bonsing BA, Busch OR, Crobach ASLP, van Delden OM, van Dieren S, Farina A, de Hingh IHJT, Hurks R, Nederend J, Shahbazi Feshtali S, Tank Y, Vahrmeijer AL, Wasser M, Besselink MG, Mieog JSD; Dutch Pancreatic Cancer Group. Intraoperative Ultrasound During Surgical Exploration in Patients with Pancreatic Cancer and Vascular Involvement (ULTRAPANC): A Prospective Multicenter Study. Ann Surg Oncol. 2023 Jun;30(6):3455-3463.
- Stibbe JA, de Barros HA, Linders DGJ, Bhairosingh SS, Bekers EM, van Leeuwen PJ, Low PS, Kularatne SA, Vahrmeijer AL, Burggraaf J, van der Poel HG. First-in-patient study of OTL78 for intraoperative fluorescence imaging of prostate-specific membrane antigen-positive prostate cancer: a single-arm, phase 2a, feasibility trial. Lancet Oncol. 2023 May;24(5):457-467.
-Baart, V.M., van Manen, L., Bhairosingh, S.S. et al. Side-by-Side Comparison of uPAR-Targeting Optical Imaging Antibodies and Antibody Fragments for Fluorescence-Guided Surgery of Solid Tumors. Mol Imaging Biol 25, 122–132 (2023).
- Tanyi JL, Randall LM, Chambers SK, Butler KA, Winer IS, Langstraat CL, Han ES, Vahrmeijer AL, Chon HS, Morgan MA, Powell MA, Tseng JH, Lopez AS, Wenham RM. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J Clin Oncol. 2023 Jan 10;41(2):276-284.
- Mieog JSD, Achterberg FB, Zlitni A, Hutteman M, Burggraaf J, Swijnenburg RJ, Gioux S, Vahrmeijer AL. Fundamentals and developments in fluorescence-guided cancer surgery. Nat Rev Clin Oncol. 2022 Jan;19(1):9-22.
- de Valk KS, Deken MM, Handgraaf HJM, Bhairosingh SS, Bijlstra OD, van Esdonk MJ, Terwisscha van Scheltinga AGT, Valentijn ARPM, March TL, Vuijk J, Peeters KCMJ, Holman FA, Hilling DE, Mieog JSD, Frangioni JV, Burggraaf J, Vahrmeijer AL. First-in-Human Assessment of cRGD-ZW800-1, a Zwitterionic, Integrin-Targeted, Near-Infrared Fluorescent Peptide in Colon Carcinoma. Clin Cancer Res. 2020 Aug 1;26(15):3990-3998.
- Boogerd LSF, Hoogstins CES, Schaap DP, Kusters M, Handgraaf HJM, van der Valk MJM, Hilling DE, Holman FA, Peeters KCMJ, Mieog JSD, van de Velde CJH, Farina-Sarasqueta A, van Lijnschoten I, Framery B, Pèlegrin A, Gutowski M, Nienhuijs SW, de Hingh IHJT, Nieuwenhuijzen GAP, Rutten HJT, Cailler F, Burggraaf J, Vahrmeijer AL. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol. 2018 Mar;3(3):181-191.
- Hoogstins CE, Tummers QR, Gaarenstroom KN, de Kroon CD, Trimbos JB, Bosse T, Smit VT, Vuyk J, van de Velde CJ, Cohen AF, Low PS, Burggraaf J, Vahrmeijer AL. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin Cancer Res. 2016 Jun 15;22(12):2929-38.
- van der Vorst JR, Schaafsma BE, Hutteman M, Verbeek FP, Liefers GJ, Hartgrink HH, Smit VT, Löwik CW, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer. 2013 Sep 15;119(18):3411-8.
- Verbeek FP, Troyan SL, Mieog JS, Liefers GJ, Moffitt LA, Rosenberg M, Hirshfield-Bartek J, Gioux S, van de Velde CJ, Vahrmeijer AL, Frangioni JV. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat. 2014 Jan;143(2):333-42.
- Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013 Sep;10(9):507-18.
8. Education
Education is a critical component of our mission at Green Light Leiden. We are committed to training the next generation of surgeons and researchers in the latest image-guided surgery techniques and technologies. Our educational contributions include:
Student Education
We are deeply committed to the education of the next generation of medical professionals. We provide courses on image-guided surgery as part of the bachelor and master programs in Medicine, Biomedical Sciences, and Medical Technology at the Leiden University Medical Center. Our curriculum integrates image-guided surgery into the broader context of medical education, ensuring that students gain a solid foundation in both the theoretical and practical aspects of this cutting-edge field. Additionally, we offer numerous internship opportunities for students to gain real-world experience in our state-of-the-art research facilities. During these internships, students work alongside our researchers and clinicians, participating in groundbreaking projects and gaining invaluable insights into the clinical applications of image-guided surgery.
…Education is a critical component of our mission at Green Light Leiden. We are committed to training the next generation of surgeons and researchers in the latest image-guided surgery techniques and technologies. Our educational contributions include:
Student Education
We are deeply committed to the education of the next generation of medical professionals. We provide courses on image-guided surgery as part of the bachelor and master programs in Medicine, Biomedical Sciences, and Medical Technology at the Leiden University Medical Center. Our curriculum integrates image-guided surgery into the broader context of medical education, ensuring that students gain a solid foundation in both the theoretical and practical aspects of this cutting-edge field. Additionally, we offer numerous internship opportunities for students to gain real-world experience in our state-of-the-art research facilities. During these internships, students work alongside our researchers and clinicians, participating in groundbreaking projects and gaining invaluable insights into the clinical applications of image-guided surgery.
ESSO Fluorescence
In partnership with the European Society of Surgical Oncology (ESSO), we offer specialized training programs in fluorescence-guided surgery. These courses are designed to provide surgeons with hands-on experience and in-depth knowledge of the latest advancements in this field.
DSFGS / ISFGS
We collaborate with the Dutch Society for Fluorescence-Guided Surgery (DSFGS) and the International Society for Fluorescence-Guided Surgery (ISFGS) to promote the dissemination of knowledge and best practices. Through conferences, workshops, and publications, we contribute to the global advancement of fluorescence-guided surgery.
Mobula Academy
Together with Mobula Academy we offer comprehensive educational e-learning programs on fluorescence-guided surgery. These programs cover a wide range of topics, including the basics of fluorescence imaging, clinical applications of this technique and its integration in the operating room. Our goal is to equip healthcare professionals with the skills and knowledge they need to improve patient outcomes using the latest image-guided surgery techniques.
10. Shaping a sustainable future: Green Light Green
At Green Light Leiden, we are dedicated not only to advancing medical technology but also to promoting sustainability in all aspects of our operations. Our "Green Light Green" initiative is our commitment to reducing our environmental impact and fostering sustainable practices. Key components of this initiative include prioritizing and promoting eco-friendly methods and materials in our research processes, contributing to the ‘Sustainable OR’ research program, and integrating sustainability principles into our educational programs to inspire the next generation of healthcare professionals to adopt green practices.